
As the molecule gets larger, the two properties diverge. Comparing molecular weight and molecular massįor small molecules, the molecular weight is often the same as the monoisotopic mass (when rounded to the nearest integer). Mass spectrometry is the only experimental technique that can distinguish between isotopes, and therefore the only technique with which the mass is more important than the molecular weight. Given the formula of a chemical species, the calculator determines the exact mass of a single isotope of that species and the relative abundance of that isotope. The monoisotopic mass is calculated using the mass of the most common isotope of each element. Accepted entry includes: chemical name, InChI, InChI key, nominal mass, or molecular formula (Hill order) KnowItAll IR, Raman, and UV-Vis spectral libraries are not included in Compound Search at this time. The online version of Molecular Mass Calculator is currently unavailable.
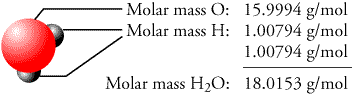
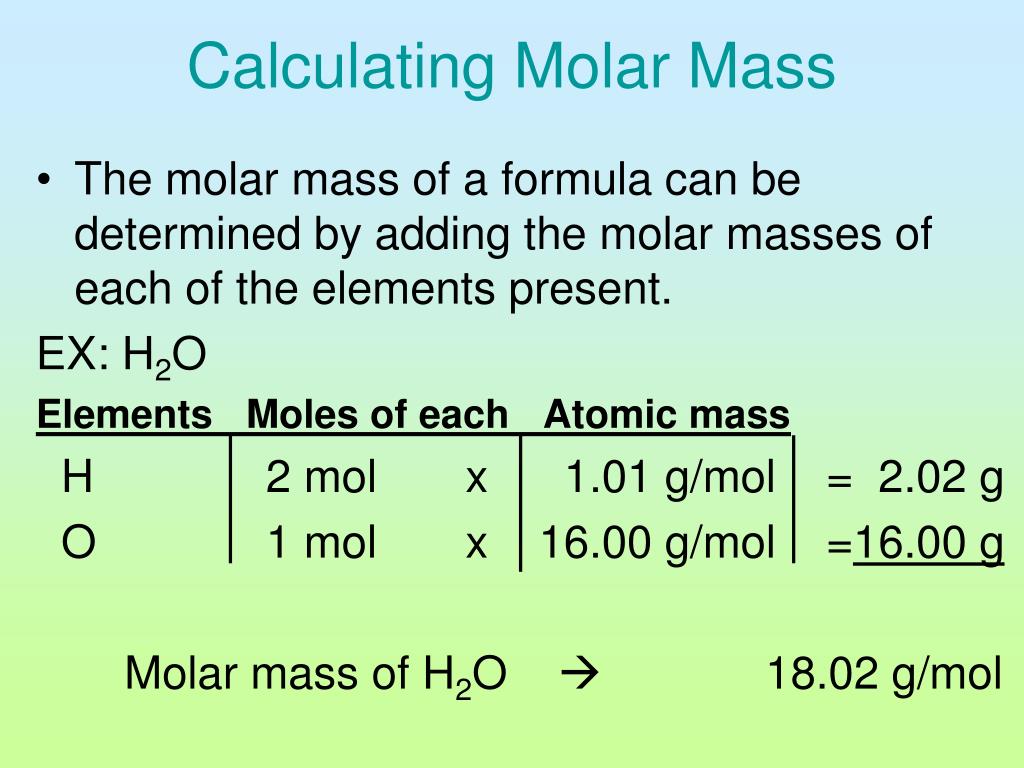
When calculating the molecular mass, individual isotopes are considered separately. The definition of molecular weight is most authoritatively synonymous with relative molecular mass. The molar mass is usually the more appropriate figure when dealing with macroscopic (weigh-able) quantities of a substance. If we round off the atomic masses to four significant figures, we get: molecular mass C6H12O6 6 (12.01) + 12 (1.008) + 6 (16.00) 180.16. The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in g/mol. The limits of the range should be separated by a comma. (The analogous property of an atom is called mass number.) Monoisotopic mass Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. Molecular Weight Search Help Search options (step 1) (Back to search) You may search for species based on molecular weight values in two ways: Specify a single value the system will search for values within 0.5 units of this value. The molecular mass is defined as the weight of a molecule relative to 12C, which is defined as exactly 12 Dalton (Da) or atomic mass units (amu). You can enter the values of any two known parameters in the input fields of this calculator and find the missing parameter. \footnotesize Yield = n starting material n product × 100%, Īnd n can be obtained from the experimentally measurable mass via Eqn. Mass to Mole Calculator This all-in-one online Mass to Mole Calculator performs calculations using the formula linking the mass of a substance, its molar mass and the molar content of the substance.


 0 kommentar(er)
0 kommentar(er)
